Better outcomes for patients may arise from a bioengineering innovation that helps heal broken bones without having the unfavourable side effects of conventional therapies, according to experts.
It could aid in the creation of novel therapies that would enable patients with cancer who have lost bone due to their illness or those who have major skeletal injuries to regenerate bone.
In developmental biology, growth factors are crucial because they serve to structure an organism’s growth from infancy to adulthood. Additionally, they aid in the body’s ability to heal from wounds by starting a convoluted chain of events that reassemble tissues.
Growth factor treatments are a potential means of assisting patients in healing by enhancing their body’s natural processes of regeneration. These therapies involve the targeted introduction of certain active proteins to promote tissue regrowth.
However, when utilised to treat bone mending, growth factor therapy may have detrimental side effects. For active proteins to be effective, large amounts of protein must be applied at the site of bone deformities or cracks. Ectopic bone development is the term for the unwanted bone formation that might result from the uncontrollably released active growth factors at the site of the implant. Other adverse effects of the therapies, such as postoperative inflammation, may also occur and may be harmful to the patient’s health.
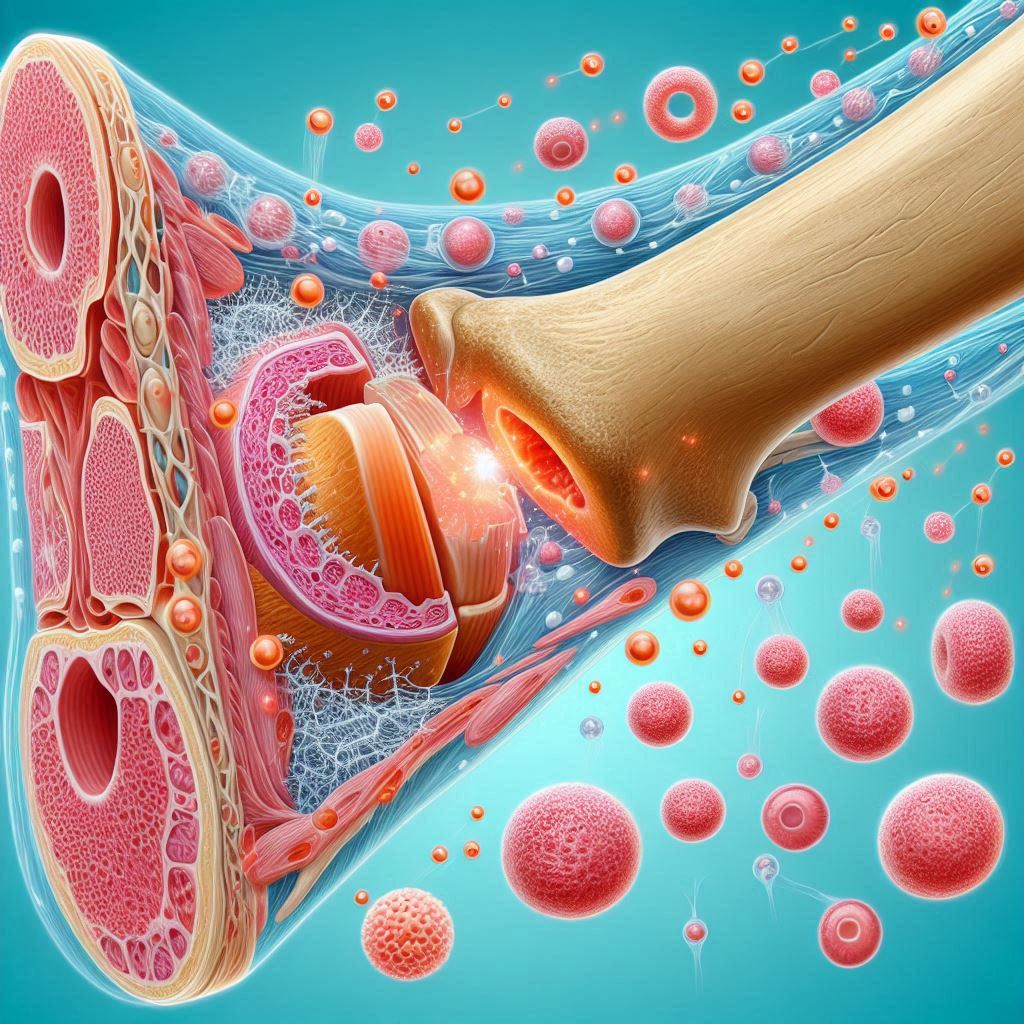
The research team led by the University of Glasgow describes their success in a new publication that was published in the journal Advanced Materials. They created a surgical implant that may be placed at the location of a bone deficiency using poly(ethyl acrylate), or PEA, a cheap polymer. The scientists were able to extract the body’s dormant growth factors and make sure they only began to function where they were needed because of the special qualities of the implant’s surface.
The team’s earlier studies have demonstrated how PEA interacts with fibronectin, a protein that is widely distributed throughout the human body and aids in cell adhesion and growth, to create nanoscale networks of the protein throughout its surface.
A portion of the fibronectin molecule is exposed as a result of the fibronectin’s structure changing as the network takes shape. The body uses certain amino acids naturally to aid in cell attachment and to store dormant proteins.
Latent transforming growth factor beta-binding protein-1, or rLTBP1, is a recombinant protein fragment that the researchers put over the fibronectin network to cause the two proteins to adhere to one another.
rLTBP1 attracts TGF-β1, a protein that stimulates the body’s growth factor cells to generate new bone tissue at low concentrations. TGF-β1 molecules get ensnared in the LAP protein complex, which renders the protein inert until it is needed to promote bone repair.
They applied rLTBP1, PEA, and fibronectin coatings on tiny plastic tubes. The ability of these implants to rebuild bone in animals with critical-sized defects was then shown. Throughout the investigation, they saw total bone defect healing.
Upon reaching the region of the bone defect, integrins, which are attachment sites on the cell surface, attached themselves to the LAP molecules that had been immobilised by rLTBP1 on the implant surface. After integrins on one side and rLTBP1 on the other mechanically pulled LAP, TGF-β1 was released, initiating its growth factor signalling activities. Only where it was necessary, regulated bone growth emerged as a result.
The biological processes that underpin this study have been understood for more than two decades, but this is the first time that they’ve been harnessed to produce this regenerative effect.
Being able to deliver immobilised proteins directly to the treatment site in this way provides much more control over how growth factors become active and start the healing process. It also works at much lower concentrations than previous treatments, helping further minimise the chances of unwanted bone growth beyond the site in need of healing.
Dr Udesh Dhawan, Research Fellow at the University of Glasgow’s James Watt School of Engineering, is the paper’s lead author.
The findings of the team are based on years of sophisticated study on bone regeneration conducted under the direction of Professors Manuel-Salmeron-Sanchez and Matthew Dalby of the University of Glasgow. One of their groundbreaking treatments prevented the amputation of a severely damaged dog’s leg in 2017.
In order to regenerate bone in the dog’s limb, the researchers used the growth factor potential of a protein known as BMP-2 that was attached to a PEA surface. The team describes in the current research how they measured the efficacy of rLTBP1 in bone regeneration using BMP-2 in a parallel control trial. Even at lesser dosages, they discovered that rLTBP1 assisted in bone regeneration equally as effectively as BMP-2.
Growth factors are very powerful tools for helping the body heal, but currently they need to be very carefully applied to prevent negative side effects cancelling out any positive therapeutic benefits. Our approach to controlling the activation of growth factors could create new opportunities for patients in the future. It could help regrow bone for patients who have lost large sections to diseases like cancer or through serious accidents, providing a much higher quality of life for them.
Professor Salmeron-Sanchez
Also Read| Atomic-level view in native mitochondria obtained using in situ microscopy
This is a new step in the right direction, but physiological systems are more interconnected than we can imagine and how this new strategy affects other crucial components of the body such as immune cells still needs to be evaluated. Nevertheless, these are very encouraging results, which suggest that this new treatment could have real benefits in clinical settings to encourage bone regeneration.
Dr Udesh Dhawan
Source: University of Glasgow News
Journal Reference: Dhawan, Udesh, et al. “Engineered Surfaces That Promote Capture of Latent Proteins to Facilitate Integrin-Mediated Mechanical Activation of Growth Factors.” Advanced Materials, vol. 36, no. 23, 2024, p. 2310789, https://doi.org/10.1002/adma.202310789.
Last Modified: